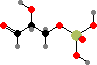
Glyceraldehyde
3-phosphate
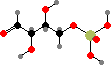
Erythrose 4-phosphate
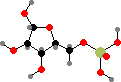
Ribose 5-phosphate
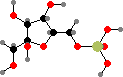
Fructose 6-phosphate
Introduction -
Common -
Bacteria -
Plantae -
Chromista -
Protozoa -
Fungi -
Animalia -
References
Nucleotides -
Amino acids -
Tetrapyrroles -
Carotenoids
Simple sugars are one of the basic starting materials for other molecules in cells. These are synthesized as phosphates; the main precursors for other compounds are C3 glyceraldehyde, C4 erythrose, C5 ribose, and C6 fructose phosphate. These and other isomers are interconverted by joining carbon chains or transferring atoms between them.




Note that –O–C–OH bridges, called hemiacetals and hemiketals, interconvert with open –OH C=O isomers and so the stereochemistry there is not fixed. If other hydroxyl groups are available the ring size can change too, and without the phosphate groups ribose and fructose would mostly occur with six-membered rings instead.
Ribose phosphate is combined with different bases to form nucleotides, which are critical as coenzymes for many different metabolic reactions. Adenosine, guanosine, uridine, and cytidine phosphate are also the units for ribonucleic acid or RNA, polymers that provide both the information and machinery for synthesis of proteins.
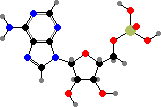
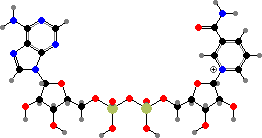
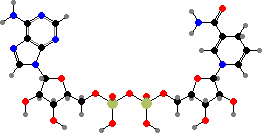
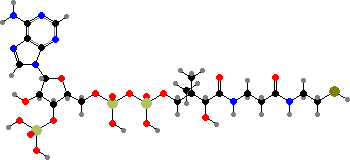
Adenosine is used as its triphosphate to provide energetic phosphate groups, or more often just energy by their hydrolysis. The monophosphate also forms part of several other coenzymes, notably nicotinamide adenine dinucleotide (NAD+ or NADH) used to carry hydrogen atoms and coenzyme A used to carry acids in the form of thioesters.
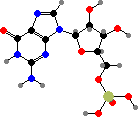
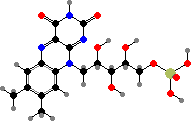
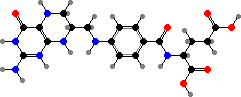
Guanosine is a precursor for flavins and pterins. Riboflavin phosphate is a group in various flavoproteins that can carry hydrogen atoms, either on its own or in a dinucleotide. It also absorbs blue light and so can act as a photosensor. The pterin derivative tetrahydrofolic acid is another coenzyme that carries single carbon groups like methylene or formyl.
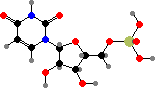
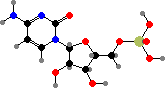
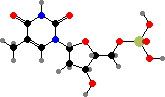
Uridine and cytidine match up with adenosine and guanosine, respectively, when nucleic acids are copied. The source copy is RNA in some viruses but for cells it is always deoxyribonucleic acid or DNA, where the component nucleotides are first converted to 2′-deoxy forms. Here uridine is also replaced by thymidine, which is given an extra methyl group.
The central hub of cellular metabolism is the tricarboxylic acid cycle, involving several simple acids that serve as precursors for other compounds. The main series consists of acetyl coenzyme A, a thioester of C2 acetic acid; and then C3 pyruvic acid, C4 oxaloacetic, malic, and succinic acids, C5 α-ketoglutaric acid, and C6 citric acid.
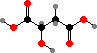
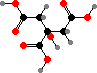
These are interconverted by addition or removal of H atoms and CO2, and in a few bacteria this can serve in fixing carbon. However that takes energy and in most organisms the steps are irreversible in the CO2-releasing direction, save C3 → C4 to replenish intermediates. New citric acid is then formed from acetyl-CoA and oxaloacetic acid.
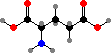
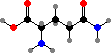
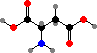
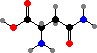
Cells take up nitrogen from ammonia to form C5 amino acids, glutamic acid and glutamine, and these are then used as sources for other compounds. C4 aspartic acid and asparagine are sometimes used to supply nitrogen as well, and in addition serve as precursors for the bases in uridine and cytidine phosphate.
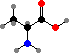
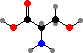
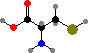
Pyruvic acid can be converted directly to alanine or indirectly to serine and sulfur-carrying cysteine. Then serine, glycine, and CO2 + NH3 can all be interconverted with addition or removal of carbon atoms by tetrahydrofolic acid, and together these provide the raw materials for forming adenosine and guanosine phosphate from sugars.
These are eight of the amino acids used to make peptides and proteins. The others are derived via more involved pathways or else required from food. Glutamic acid is a precursor for proline and arginine, and aspartic acid for methionine, threonine, and isoleucine. Lysine may be formed from either aspartic or α-ketoglutaric acid.
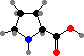
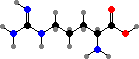
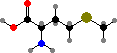
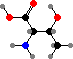
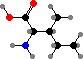
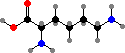
Combination of pyruvic acid units gives leucine and valine. Finally there are the four aromatic amino acids, phenylalanine, tyrosine, tryptophan, and histidine, formed by combinations of sugar phosphates. These twenty universal types are then assembled into polymers by ribosomes, with the sequence determined by RNA.
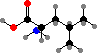
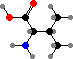
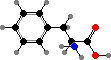
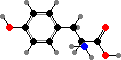
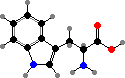
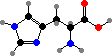
The behaviour of proteins depends on how they are folded, the result of different interactions between the amino acid side groups. Many types are also further modified, and notably cysteine residues may form disulfide links between chains. The results are a vast diversity of different enzymes, structural materials, signal molecules, toxins, and so on.
Tetrapyrroles are important both in absorbing light and forming metal complexes, including some of the key compounds making energy available to cells. Most are derived via protoporphyrin. This combines with ferrous ions to form heme, which is found throughout the living world as the prosthetic group of various proteins.
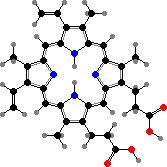
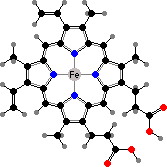
The most widespread of these are cytochromes, where heme or derivatives are involved in carrying electrons between metabolism and inorganic agents like oxygen. It also appears in catalases and peroxidases, enzymes to break down peroxide ions, and in many vertebrates, worms, and other animals in red hemoglobins that transport or store oxygen.
Breakdown of heme produces biliverdin. This is common as a waste product, but is also the active group in the light-sensing proteins of some bacteria, a precursor of other bilins, and a blue-green pigment in insects and other animals. Protoporphyrin and related compounds can also give red-brown colours but are photosensitizers, so rare except in cases like shells.
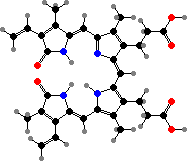
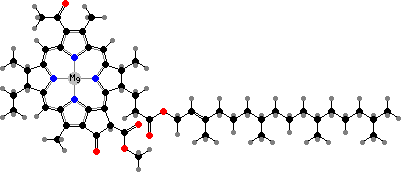
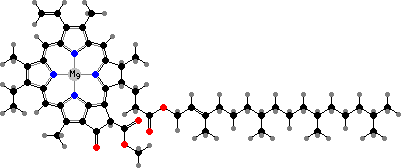
Most photosynthesis relies on green tetrapyrroles with magnesium ions as primary antenna pigments, which transfer light energy to electrons. Anoxygenic bacteria have bacteriochlorophyll aP or other bacteriochlorins, while cyanobacteria, plants, and other algae share chlorophyll a, a phytochlorin. These are accompanied by various other light-gathering pigments.
Carotenoids are a large group of pigments derived as terpene dimers. They occur in all photosynthetic organisms, where they are critical in quenching excess energy absorbed by the antenna pigments but may also aid in light-gathering. They are also found as antioxidants in a variety of aerobes, and are a major part of the visual appearance of many organisms.
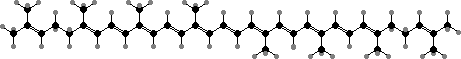
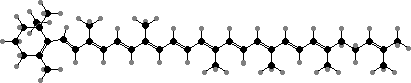
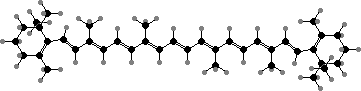
The majority of carotenoids have 40 carbon atoms with lycopene, γ-carotene, and β-carotene as both common types and successive precursors for other acyclic, monocyclic, and bicyclic types. The rings are tilted and so twist the double bonds from the plane of the others, shifting the colour from red in lycopene to orange-yellow.
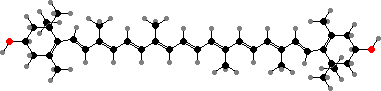
Most other types are polar including a variety of hydroxy-carotenoids, for instance yellow zeaxanthin in many groups, and keto-carotenoids, like red-orange canthaxanthin and astaxanthins. The last are especially common in animals, which accumulate or modify carotenoids from their diet, often as esters or in variously coloured protein complexes.
Cyclic carotenoids also serve as precursors for retinal, which combines with proteins called opsins to form light-sensing pigments in halobacteria, some flagellate algae, and the eyes of animals, among others. Depending on the opsin the absorption can be anywhere from ultraviolet to red, with different colours visible to different groups.